COVID-19 Vaccination
COVID-19 Vaccine
DCHHS Immunization Clinics
COVID-19 Vaccines are Available for Children 6 months+
Monday - Friday, 8:30am - 3:30pm
APPOINTMENTS OR WALK-INS AVAILABLE
(972) 692-2780
Comirnaty (Pfizer) - ages 12yrs and older | Pfizer - ages 6mo to 11yrs | Spikevax (Moderna) - ages 12yrs and older | Moderna - ages 6mo to 11yrs
available at all clinic locations.
* $25 Walmart gift card for any Dallas County resident who gets a COVID-19 vaccination (while supplies last) *
REMINDER: If you are getting an additional dose please bring your vaccination card with you.
Dallas County Health and Human Services offers COVID vaccinations to children and adults who are eligible
Eligibility for adults:
Individuals who are 19 years of age and older are eligible to receive the Covid 19 immunizations under the Adult Safety Net (ASN) Program at Dallas County Health and Human Services:
- Uninsured is defined as a person who does not have Medicare, Medicaid, or private insurance.
- Underinsured is defined as a person who has health insurance, but the insurance does not cover vaccine costs (i.e., COVID-19 vaccines); a person whose medical insurance does not provide first-dollar coverage (i.e., copay-free coverage) of the COVID-19 vaccinations.
- First-dollar coverage is defined as health care services, such as COVID-19 vaccinations, covered pre-deductible and without cost-sharing.
Eligibility for children:
Clients eligible to receive (TVFC) immunizations at Dallas County Health and Human Services are:
- Medicaid eligible
- Uninsured: A child who has no health insurance coverage
- American Indian or Alaskan Native
- Enrolled in CHIP
- Underinsured*
*A child who has commercial (private) health insurance, but coverage does not include vaccines; a child whose insurance covers only selected vaccines (TVFC-eligible for non-covered vaccines only).
DCHHS Stemmons Immunization Clinic
2377 N. Stemmons Freeway, #159 (first floor), Dallas, TX 75207
Oak Cliff Branch Immunization Clinic
1113 E. Jefferson Boulevard, Suite 200, Dallas, TX 75203
John West Branch Immunization Clinic
3312 N. Buckner Boulevard, Suite 200, Dallas, TX 75228
Carrollton Farmers Branch Immunization Clinic
2774 Valwood Parkway, Farmers Branch, TX 75234
Irving Branch Immunization Clinic
440 S. Nursery Road, Irving, TX 75060
Richardson Branch Immunization Clinic
1411 W Beltline Rd, Richardson, TX 75080
Clínicas de Vacunación de DCHHS
Las vacunas COVID-19 están disponibles para niños mayores de 6 meses
lunes a viernes de 8:30am a 3:30pm
NO SE NECESITA UNA CITA
(972) 692-2780
Comirnaty (Pfizer) – personas de 12 años en adelante | Pfizer - 6 meses hasta 11 años | Spikevax (Moderna) - personas de 12 años en adelante | Moderna - 6 meses hasta 11 años
están disponibles en todas las clínicas.
* Residentes que se vacunen con cualquier dosis recibirán una tarjeta de regalo de Walmart de $ 25 (dependiendo de disponibilidad) *
RECUERDE: Si va a recibir una dosis adicional traiga su tarjeta de vacunación.
El Depto. de Salud y Servicios Humanos ofrece vacunas del COVID a niños y adultos que sean elegibles
Elegibilidad para adultos:
Las personas mayores de 19 años son elegibles para recibir las vacunas contra el Covid 19 bajo el Programa de Red de Seguridad para Adultos (ASN) en el Departamento de Salud y Servicios Humanos del Condado de Dallas:
- Sin seguro médico se define como una persona que no tiene Medicare, Medicaid o seguro privado.
- Seguro insuficiente se define como una persona que tiene seguro médico y el seguro no cubre los costos de las vacunas (es decir, vacunas contra el COVID-19) ni tampoco ofrece cubrir los costos del deducible o copagos para recibir la vacuna del COVID-19.
- La cobertura del primer dólar se define como servicios de atención médica, como vacunas contra el COVID-19, cubiertos antes del deducible y sin costos compartidos.
Elegibilidad para niños:
Los clientes elegibles para recibir vacunas (TVFC) son:
- Elegibles para Medicaid
- Sin seguro: un niño que no tiene cobertura de seguro médico
- Indio americano o nativo de Alaska
- Inscrito en CHIP
- Seguro insuficiente*
*Un niño que tiene seguro médico privado, pero la cobertura no incluye vacunas; un niño cuyo seguro cubre solo vacunas seleccionadas (elegible para TVFC solo para vacunas no cubiertas).
Clínica de Vacunación DCHHS Stemmons
2377 N. Stemmons Freeway, #159 (first floor), Dallas, TX 75207
Clínica de Vacunación Oak Cliff
1113 E. Jefferson Boulevard, Suite 200, Dallas, TX 75203
Clínica de Vacunación John West
3312 N. Buckner Boulevard, Suite 200, Dallas, TX 75228
Clínica de Vacunación Carrollton Farmers
2774 Valwood Parkway, Farmers Branch, TX 75234
Clínica de Vacunación Irving
440 S. Nursery Road, Irving, TX 75060
Clínica de Vacunación Richardson
1411 W Beltline Rd, Richardson, TX 75080
Find a Vaccine Location Near You
Pharmacies and clinics across Dallas County are administering the COVID-19 vaccine. To find a vaccine location near you, visit https://www.vaccines.gov/.
Encuentre vacunas contra el COVID-19 cerca de usted
Las farmacias y clínicas a través del condado de Dallas están administrando la vacuna COVID-19. Para encontrar un sitio para vacunarse cerca de usted, visite https://www.vacunas.gov.
For questions about appointments from DCHHS through Luminare, please call the DCHHS COVID Hotline at (972) 692-2780 or email CovidVaccine@DallasCounty.org.
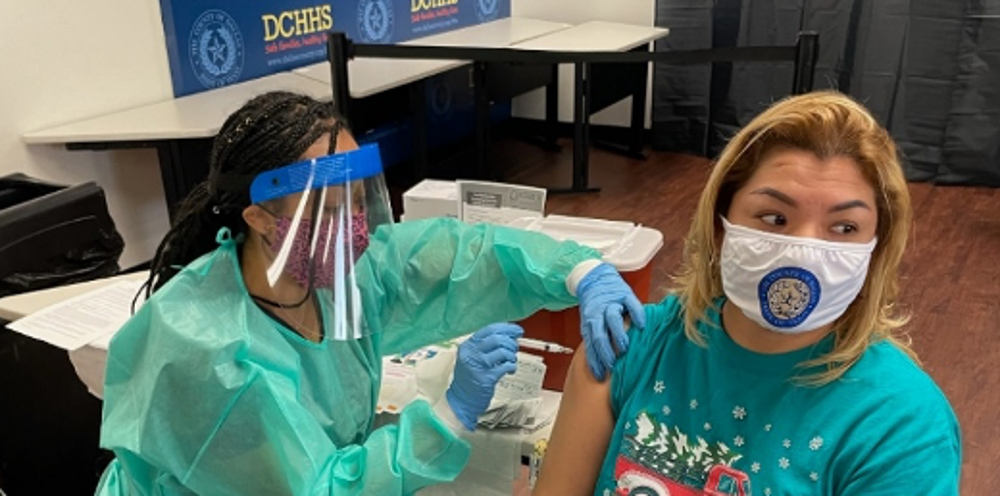
Who is eligible for a vaccine?
Everyone ages 6 months and older is now eligible to receive a COVID-19 vaccine in Texas. We strongly encourage all eligible residents to make an appointment for the COVID-19 vaccine.
What are the Vaccination Requirements for Minors?
Children 6 months and older may be vaccinated with parents/legal guardians consent.
What documentation do I need to get the vaccine?
You will need to provide a photo ID, such as a driver’s license, passport, work ID, parish ID, library card, or other form of photo ID.
Do I need to be a U.S. citizen in order to receive the vaccine?
No. The vaccine is available to anyone who wants it. No questions about citizenship status will be asked.
How do I get my second dose?
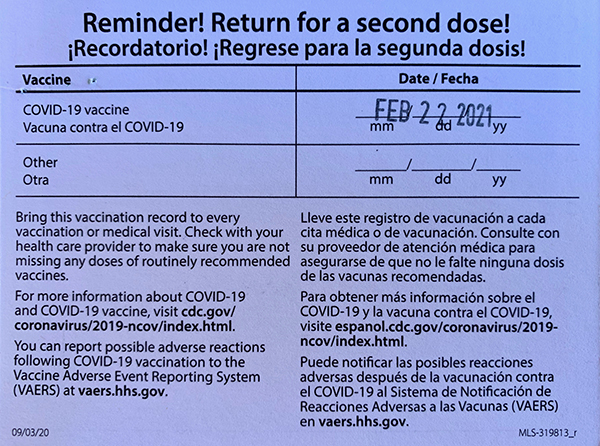
If you received your first COVID-19 vaccine dose through Dallas County Health and Human Services (DCHHS), you are eligible for your second dose through DCHHS. We will send you an email and/or text message to schedule an appointment for your second dose. You may also go to another location to receive your additional doses. Additional locations can be found at vaccines.gov.
Where else can I receive a COVID-19 vaccine?
You can visit vaccines.gov to find many other locations across North Texas.
What is the status of the COVID-19 vaccines that are being developed?
Many vaccines have received either final FDA approval or Emergency Use Authorization.
How effective are the approved vaccines?
The vaccines continue to substantially reduce the risk of severe illness and hospitalization.
How will we know these COVID-19 vaccines are safe?
The U.S. Food and Drug Administration has a known and proven process for the verification of vaccines, and while these COVID-19 vaccines have been made available quickly, no step in the safety and efficacy process was skipped. The FDA issued EUAs for the first COVID-19 vaccines, only after enough scientific data was shown to indicate the vaccines safety and efficacy in a clear and compelling manner.
The current vaccines, even those with EUAs, continue through a trial phase, where they are tracking their volunteers to learn more about the long-term outcomes of taking the vaccine.
Can the vaccine give you the virus?
None of the COVID-19 vaccines authorized for use in the United States contain the live virus that causes COVID-19, which means they cannot give someone COVID-19. You may experience symptoms after receiving the vaccine. This is a normal response and is a sign that the body is learning to recognize and is building protection against the virus that causes COVID-19.
It is possible for someone to be infected with COVID-19 prior to receiving the vaccine and thus they would be contagious and could still test positive on a COVID-19 diagnostic PCR, or rapid test. It is also possible a person could be infected with the virus that causes COVID-19 just after vaccination and still get sick. This is because it typically takes a few weeks for the body to build immunity (protection against the virus that causes COVID-19) after vaccination. An uninfected vaccine recipient however would not test positive on a PCR or rapid test but could test positive on an antibody-based test.
What companies are manufacturing the COVID-19 vaccine, and how are the vaccines different?
| Vaccine Manufacturer | Technology |
| Pfizer | m-RNA |
| Moderna Therapeutics | m-RNA |
| Johnson & Johnson | Viral Vector (non-replicating) |
Do I need a vaccine if I already had COVID-19?
Yes. The vaccine is recommended for people who previously have been infected with COVID-19. Vaccination of persons with current SARS-CoV-2 infection should be deferred until the person has recovered from acute illness and they can discontinue isolation. While there is no minimum interval between infection and vaccination, current evidence suggests reinfection is uncommon in the 90 days after initial infection. Persons with documented acute SARS-CoV-2 infection in the preceding 90 days may delay vaccination until near the end of this period, if desired.
Can I choose which vaccine I want to take?
Yes, you can select which vaccine you want. You can see which location have which vaccine by visiting vaccines.gov.
Once people start taking the COVID-19 vaccine, will we need to keep wearing masks and social distancing?
Individuals should still take precautions based on their personal risk. https://www.cdc.gov/coronavirus/2019-nCoV/index.html
Can my child get the COVID-19 vaccine?
Vaccines are approved for individuals 6 months or older.
Will the COVID-19 vaccines require special handling?
Each vaccine has different storage and preparation requirements. Public Health staff who handle vaccines are trained on storing, handling and preparing them safely to ensure the viability of every vaccine dose.
What will the COVID-19 vaccine cost?
The vaccine is free. Operation Warp Speed, a federal program, is paying all the costs associated with vaccinations.
Is getting a COVID-19 vaccine immunization mandatory?
COVID-19 vaccinations are voluntary, but we strongly recommend all eligible persons receive the vaccine.
How many doses of vaccine will I need?
Most vaccines require at least 3 doses to stay up to date. Visit our Health Guidance page - here - to see dose guidelines.
How long do I have to wait between doses?
Depending on the vaccine you receive, there may be a 21-28 days between the first and second dose and then a few months until you are eligible for booster doses. When you receive the first dose, it is important that you wait for the designated time and then get the second dose. The effectiveness of the vaccine is highest when the doses are spaced appropriately. Information will be provided to everyone who receives the COVID-19 vaccine to ensure they receive the correct second dose. To learn what you will need to do to stay up to date with all doses, please visit https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html.
Is there any proof we need to show if we’ve had a COVID-19 vaccine shot?
Those receiving COVID-19 vaccine will have the immunization noted on their official IMMTRAC vaccine record.
What if I lost my COVID-19 vaccination card or need a replacement?
You may obtain an IMMTRAC print out of your COVID vaccination at any of Dallas County Health and Human Services clinics. Monday thru Friday 8am - 4 pm:
https://www.dallascounty.org/departments/dchhs/clinical-services/immunization-clinics.php.
If you are not able to go in-person, you may request your record through the Department of State Health Services by fax or mail. Additional information about this process is available here: https://www.dshs.texas.gov/immunize/immunization-records.aspx.
QUICK LINKS
LOCATIONS
EMPLOYEES
-
You must be on the network to see these links.

